
This post is going to combine some history with praxis. If you just want to see how I make distillations with my pot still, jump ahead for directions. It’s interesting though, especially since distillation, which was once probably the most common way of producing home medicines, has entirely been passed over for more modern methods like making tinctures. Which is sad because all you need to preserve plants as distillates is some water and a glass retort.
While you might have been told that the Arabs invented distillation, that is not entirely the case. Some historians credit them with perfecting the equipment that made distilling alcohol possible, (copper coil) but even that has been a source of great historical debate.
Distillation has been around for much longer than that, though. Simple distillation apparatuses used for extracting distillates and oils from plants have been discovered at various archeological excavations including Tepe Gawra (Mesopotamia 3500 BCE) the Mohenjo Daro (Indus Valley ca. 3000 BCE) and several Chinese sites dating back to 2000BCE. The oldest methods involved simmering plants in liquid and using a lid to collect the liquid that evaporated off which I refer to as the distillate.
The Alexandrian perfumers’ guild is one of the first documented groups of alchemists. In the first century BCE, they were using simple distillation equipment to distill floral essences which seem to have included rosewater, for which Arabic perfumers were previously credited.
In a fourth-century manuscript written by guildmember Zosimus of Panapolis, there was this rough sketch of the equipment they used. This photo is from a French translation of that document. In his manuscript, he credits a woman named Maria Hebraea with inventing the system and instructs people on how to use it.
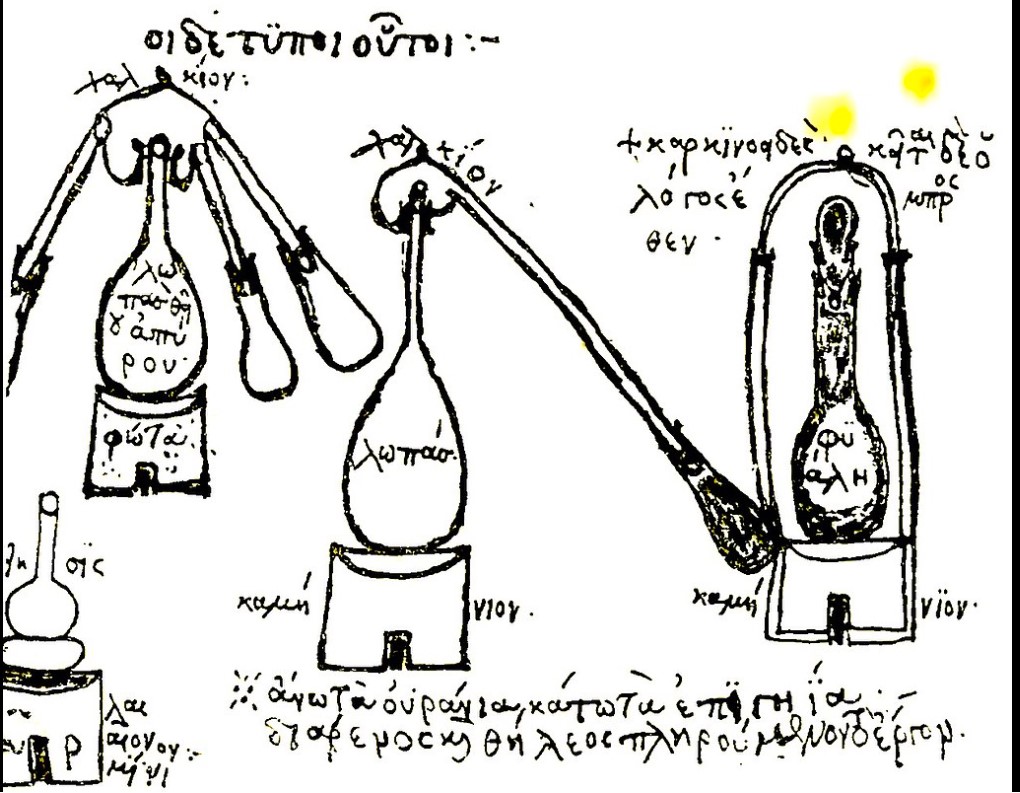
Another early illustration of a glass vase holding simmering liquid placed over a fire and covered with a conical top comes from a tablet found in the Keos, Crete excavation.
The Arab word alembic is derived from the earlier Greek word ambix that described this vessel with its cone top. Aristotle wrote instructions on how to distill seawater using this device and Pliny and Dioscórides both wrote about using the process to extract mercury from the mineral cinnabar by placing a helmet-shaped vessel over steam to catch it.
There’s no evidence that the ancients made distilled alcohol for casual drinking. Arabic scientists are given credit for inventing alembics that were coil-cooled and consequently able to produce more volume of alcohol but it seems to have been viewed as a medicine by them.
It was likely the Al Andalusians who passed the technology along to the Irish via trade networks. We don’t know exactly when, but we do know that the Irish were at it long before the rest of Western Europe. The English were taxing them for making whiskey by 1276.
There is a little confusion around the term used for whisky Uisce Beatha meaning “water of life” which was distilled from barley mash (whiskey) and the Latin aqua vite meaning “water of the vine” which is distilled from grapes (brandy). You will also find receipts for making Usquebaugh (an anglicized form of Uisce beatha) in manuscript receipt books, but it’s a cordial water and nothing like whiskey.
The first documentation that confirms the use of barley malt to brew whiskey is in the Exchequer Rolls of 1494, King James IV of Scotland awarded one Friar John Cor a large amount of malt for making aqua vitea. I am going to link to a Latin version Rotuli scaccarii regum Scotorum so you can see that text.
The definitive Western European sourcebook was Liber de arte distillandi, written by Hieronymus Brunschwyg in 1500 and translated into English by Laurens Andrewe in 1527. According to this text, aqua vite was made by distilling wine made from grapes and he extolled its medicinal values at length.1
I think that aqua vite and aqua vitea were far too similar in a time when there was no such thing as standardized spelling and that it all got jumbled up in 19th century translation.

In medieval times, households of the wealthy classes had small distillation apparatuses made of various materials to make medicines and perfumes for their home use. Not everyone who made distillates owned a still. Here you can see directions from a French manuscript for making rosewater without an alembic from 1393.2
TO MAKE ROSE WATER WITHOUT A LEAD ALEMBIC, take a barber’s basin, and fold a kerchief longwise across the opening like a drum, and then put your roses on the kerchief, and over the roses set the bottom of another basin containing hot ashes and live coals.
TO MAKE ROSE WATER WITHOUT ALEMBIC OR FIRE, take two glass bowls, and do as said earlier, and in place of ashes and coals, put it all out in the sun: and the heat of the sun will make the rosewater form.
I believe the reason that simple waters were so popular is that all you needed for preservation was water and the plant material. People who might not have had the luxury of having access to a fire had tricks for making them. Roger Bacon (aka Dr. Mirabilis) mentioned setting up your equipment in balneo with sand in vessel A and leaving it in the sun to let it rectify.3
Many wealthy homes had small batch distillation equipment for making these distillates like the Hamm House still below. In his chapter on distillation in Delights for Ladies published in 1600, Hugh Plat mentions pewter, brass, copper, glass, and lead as materials for “limbecks.” Plat had preferences about which material he used for making each preparation. For example, he recommended copper for making cinnamon water but used pewter for Usquebath.

There’s a reason for this. Most metals are not inert which means they chemically react with one substance or another. For example, copper reacts with acids and sulfur bonds. This can be good when using copper to remove sulfites from wine, but not so great if you want sulfur in your final product. In that case, you would use glass.
I decided that I don’t want to have to think that hard and picked a small stainless-steel pot still that can just be used on the stovetop. Stainless steel is a nice modern metal that is inert even if it doesn’t conduct heat as well as copper, but it still has copper tubing. If I don’t want copper to interact with my distillate, I have glass labware to fall back on. Someday when I get a proper retort, I will write about that.
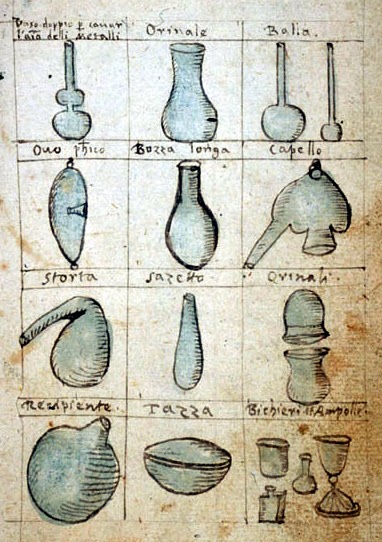
Row one: double vase, a urinal, 2 flasks ,
Row two: philosopher’s egg, flask, alembic
Row three: retort, bottle, urinal with cover,
Row four: receiving flask, a saucer, cups and ampules.
General Directions for Using a Pot Still for Home Distillations
Some stills like mine come with a column that has holes in the bottom and attaches above a boiler. If yours does not have this and you feel strongly about using a column, you can improvise. I always place a stainless-steel rack on the bottom of my boiler to keep the plant material from touching the bottom and place the plant material in a stainless-steel steaming basket. This way the plant material is not in danger of scorching or sticking.


After you have placed the plant material in the basket this way, fill your boiler half full of water. The exact amount is going to vary depending on the amount it holds. If you are not distilling something acidic, it helps to add just a bit of citric acid to your water because you want it to be slightly acidic. An ideal pH is somewhere between 5.5 and 6.
Attach the condenser to your kettle. Connect the water lines to the condenser. The top line should then be connected to your faucet. The bottom line is your return line. If you hate wasting water like I do, you can collect the water in a bucket and use it for watering plants or make yourself a circulating system with an aquarium pump.

Place your boiler on a heat source and turn it on. When the temperature on the boiler temperature probe reads around 180°F, turn on the cold water slowly until the water in the condenser is covered. ETA 22 Jan 2023: I finally made my recirculating pump

As the cold water circulates around the coils it condenses the steam and the resulting liquid is your distillate. Catch your distillate in small amounts in a non-reactive container with a tapered lid. I use labware made for this.
You should be able to hear when your water is getting low in the boiler. Then remove the still from the heat. When the liquid in the boiler cools, you can strain it and use it for baths or making syrups.
Pour the distillate into a larger airtight container to settle. Cap the container that you are storing your distillate in tightly to prevent evaporation. It will appear cloudy at first but as it cools the aromatic constituents will mostly be floating on top.
The essential oils will float on top of the solution as it cools, and you can just use a dropper to siphon it off the top. I am not really into essential oils. I dilute them anyway, so I just bottle my hydrosols in 12 oz beer bottles and keep them in a cool place.
Most of you aren’t going to have bottling equipment so use dark-colored, airtight, glass containers to bottle your hydrosols and essential oils. Clearly label them with the type of preparation and distillation date.
After the still cools down, clean the entire unit with vinegar and water. These aromatic constituents are very strong and can linger, so you want to be sure to thoroughly clean the unit. I find it useful to set the equipment in the sun to dry.
References
- Brunschwig, Hieronymus. Liber de Arte Distillandi; English. Translated by Andrewe, Laurens. London, England. 1527 ↩︎
- Montigny, Guy (presumed). Le Ménagier de Paris. Translated by Hinson, Janet, 1393. ↩︎
- Bacon, Roger. This Boke Doth Create All of the Beste Waters … Edited by Wyer, Robert. 1551 reprint. London, England: Imprynted by me Robert wyer, 1294. and Gesner, Conrad. The Treasure of Euonymus Fornaces, and Vessels, Required in This Art… Translated by Peter Morwen. London, England: By Iohn Daie, dvvelling ouer Aldersgate, beneath Saint Martines. 1559. ↩︎
One thought on “Making Distillates at Home”
Comments are closed.